Abstract
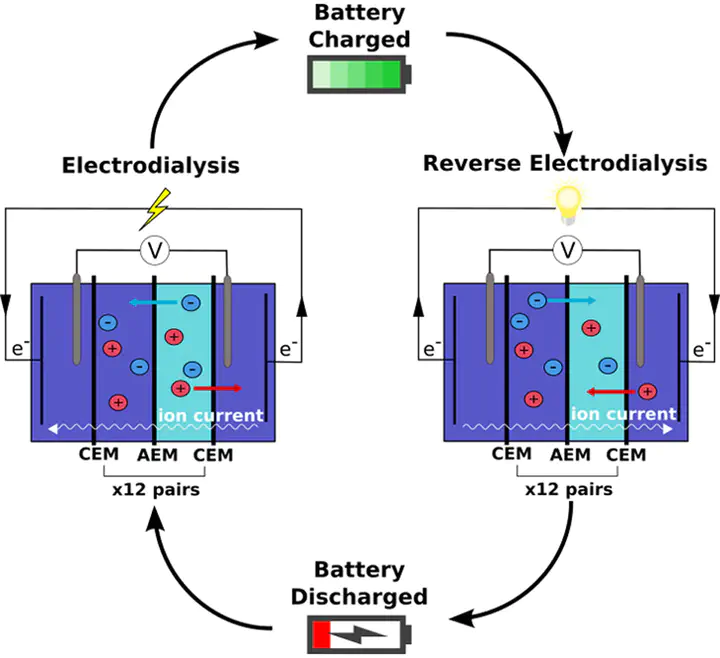
Reverse electrodialysis has long been recognized as a tool for harnessing free energy from salinity gradients but has received little attention for its potential in energy storage applications. Here we present the experimental and modeled performance of a rechargeable electrodialytic battery system developed for the purpose of energy storage. Experimental round-trip energy efficiency ranged from 21.2% to 34.0% when cycling the system between 33% and 40-90% state of charge. A mass transport model based on chemical thermodynamics is also proposed to describe the system’s performance. Results indicate that, upon model calibration, the model effectively predicts experimental values. Experimental and modeled results suggest that the membrane resistance and osmosis are the primary sources of ohmic and faradaic energy losses, respectively. The results demonstrate that a functioning battery can be constructed using typical reverse electrodialysis stack components. Future improvements in membrane technology and optimization of the system chemistry offer promising avenues to improve the power density, energy density, and round-trip energy efficiency of the process.